In a nutshell: biomedical research data management
Digital transformation has significantly increased the importance of research data management. Research data in the biomedical field, including clinical and epidemiological research, are central to generating (health) knowledge, making recommendations and developing new therapies and technologies. This change poses new challenges for professionals, policy makers, funders and educational institutions in particular. At the heart of these challenges is the need to equip stakeholders with the necessary knowledge about research data management.
What does FAIR mean for research data management?
FAIR stands for Findable, Accessible, Interoperable and Reusable. Managing research data according to the FAIR principles enables researchers and data users to make research data findable, accessible and reusable, while complying with legal and ethical requirements. The FAIR Principles are now part of the good scientific practice of many scientific institutions and professional societies.
Further information on the FAIR principles can be find here.
Why should I make my research data FAIR?
By making your data FAIR, other researchers have the opportunity to re-use it. On the one hand, this re-use reduces the amount of redundant research and makes it possible to carry out analyses with large numbers of cases (e.g. meta-analyses). In accordance with good scientific practice and Creative Commons licences, reusers (e.g. authors) are required to cite your data when you make it available. FAIR data can help make your research more visible.
How can I make my research data FAIR?
Research data have a continuous life cycle. From project planning to project completion, you can contribute at every stage to making your data findable, accessible, interoperable and reusable, i.e. FAIR. NFDI4Health can support you in this process with training and services. The individual steps are illustrated in the data life cycle:
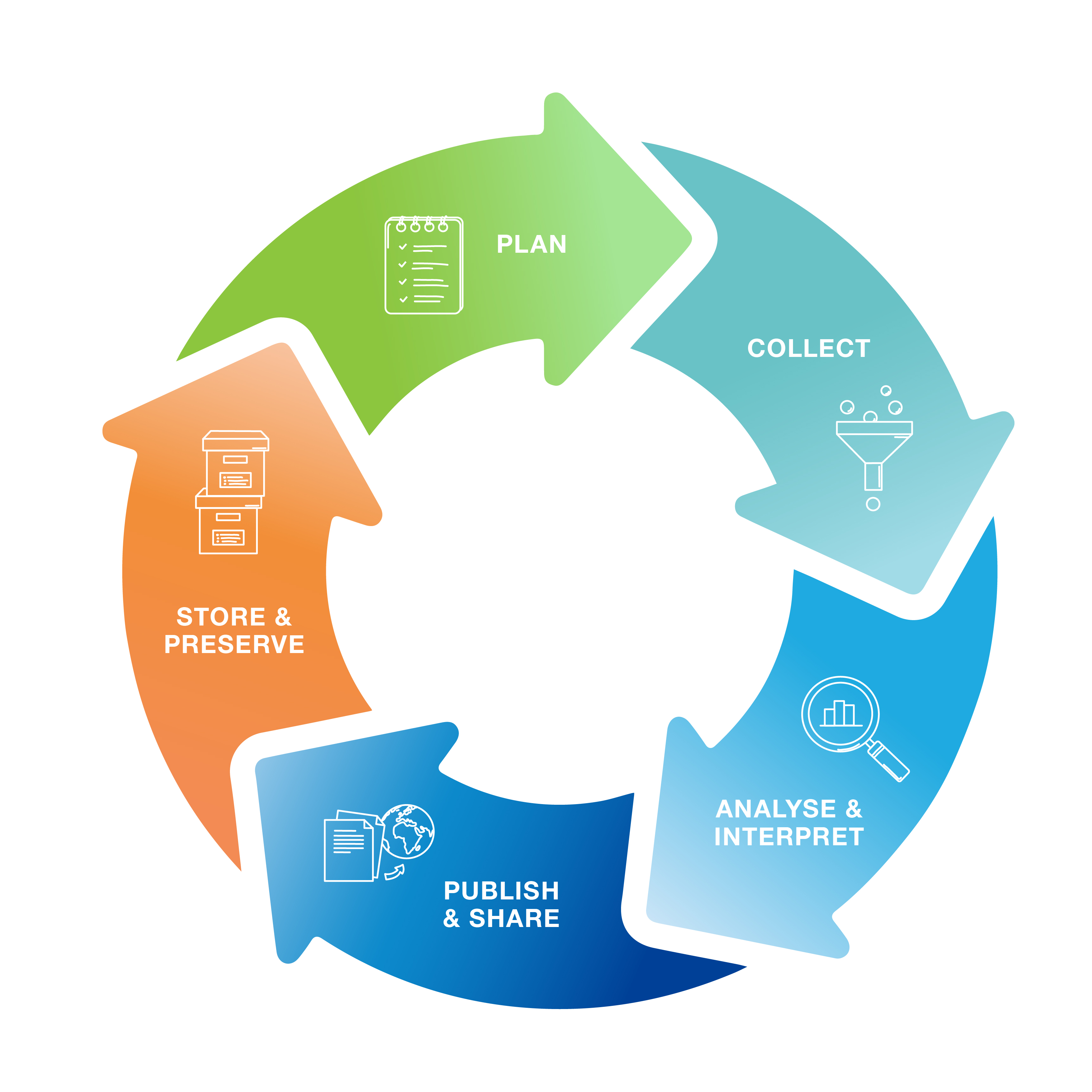
PLAN: What should I consider when planning a research project?
FAIR data management starts even before the respective projects begin. New research ideas are quickly found. However, in addition to planning research objectives, some funding bodies also expect fair project planning, including data handling. The training and services of NFDI4Health can provide you with targeted support. You can also use our Research Data Management Plans, which were specifically developed in collaboration with scientists from clinical and epidemiological studies.

COLLECT: What should I bear in mind when collecting data?
In the digital age, a large amount of research data is collected in every research project. The interoperability of the data therefore plays a major role right from the data collection stage. Make sure that established standards and ontologies are used in your specialist area. Use machine-readable data formats. This is crucial if data from different sources is merged or different software and tools are used. The creation of metadata, i.e. data that describes the study and the data collected in it, is also essential for the FAIRification of your own research work. Every study should have a so-called data dictionary.

ANALYZE & INTERPRET: What is the role of data management in data analysis?
In biomedical research, standard procedures (e.g. use of classification systems and indices, statistical methods) for data analysis have been in place for many years. However, in addition to describing the methods in scientific articles, it is desirable to document the variables created during the analysis. The data dictionary associated with the study or research project should therefore be kept up to date. A data dictionary template for clinical and epidemiological studies can be found in the NFDI4Health publication policy.

PUBLISH & SHARE: How do I make my research data discoverable?
In addition to publishing the results of a research project, researchers should also consider publishing their research data and associated metadata. Making rich metadata available in a repository allows other researchers or interested parties to find these datasets. For this reason, we have developed a platform for finding and publishing health research data in NFDI4Health, the German Central Health Study Hub. On the one hand, this repository allows scientists and data owners to publish their project- or study-specific metadata, documents and research data. On the other hand, data can be found and re-used by researchers.

STORE & PRESERVE: How do I make my research data accessible in the long term?
Research data should be stored in formats and on platforms that allow authorized users to access it long after it has been created. Appropriate authentication and authorisation protocols ensure that data remains both secure and accessible. This means that research data is not lost when studies are completed. Beyond its original purpose, data should be structured and documented so that it can be used by others for further research. Clear licensing and data dictionaries play a crucial role in this. For this reason, we have developed a platform for finding and publishing health research data in NFDI4Health, the German Central Health Study Hub. On the one hand, this repository allows scientists and data owners to publish their project- or study-specific metadata, documents and research data. On the other hand, data can be found and re-used by researchers.
Repositories: trustworthy storage solutions
Biomedical data repositories e.g. the NFDI4Health German Central Health Study Hub offer structured storage solutions with built-in tools for data search, validation, and analysis.
Data protection: beyond just security
Protecting data in the biomedical field isn't merely about preventing unauthorised access. It also involves ensuring that personal patient data remains anonymous and that ethical standards are met, while adhering to regulatory guidelines such as GDPR (General Data Protection Regulation) or HIPAA (Health Insurance Portability and Accountability Act).
Access to data: ethical and legal facets
Forschungsdatenportal für Gesundheit (FDPG): Central contact point for researchers who want to carry out a research project with routine data from German university medicine and other affiliated locations.
Use & Access Committees: These committees evaluate the intent behind data access requests. Their aim is to balance open science with ethical research practices.
Embracing and understanding these facets of Biomedical RDM ensures that researchers not only comply with global standards but also pave the way for innovative, ethical, and impactful research.
 English
English  Deutsch
Deutsch 